Thermodynamics Can Be Used to Describe Biochemical Reactions
The overall reaction thermochemistry can be calculated exactly by combining the BDEs for the bonds broken and bonds formed ie ΔH BDEbonds broken BDEbonds made The bonds made part of the equation is negative because it represents the opposite of bonds broken the BDE. This chapter describes the mechanisms by which phosphate and sulfate monoesters undergo uncatalyzed and enzyme-catalyzed hydrolysis by phosphatases.
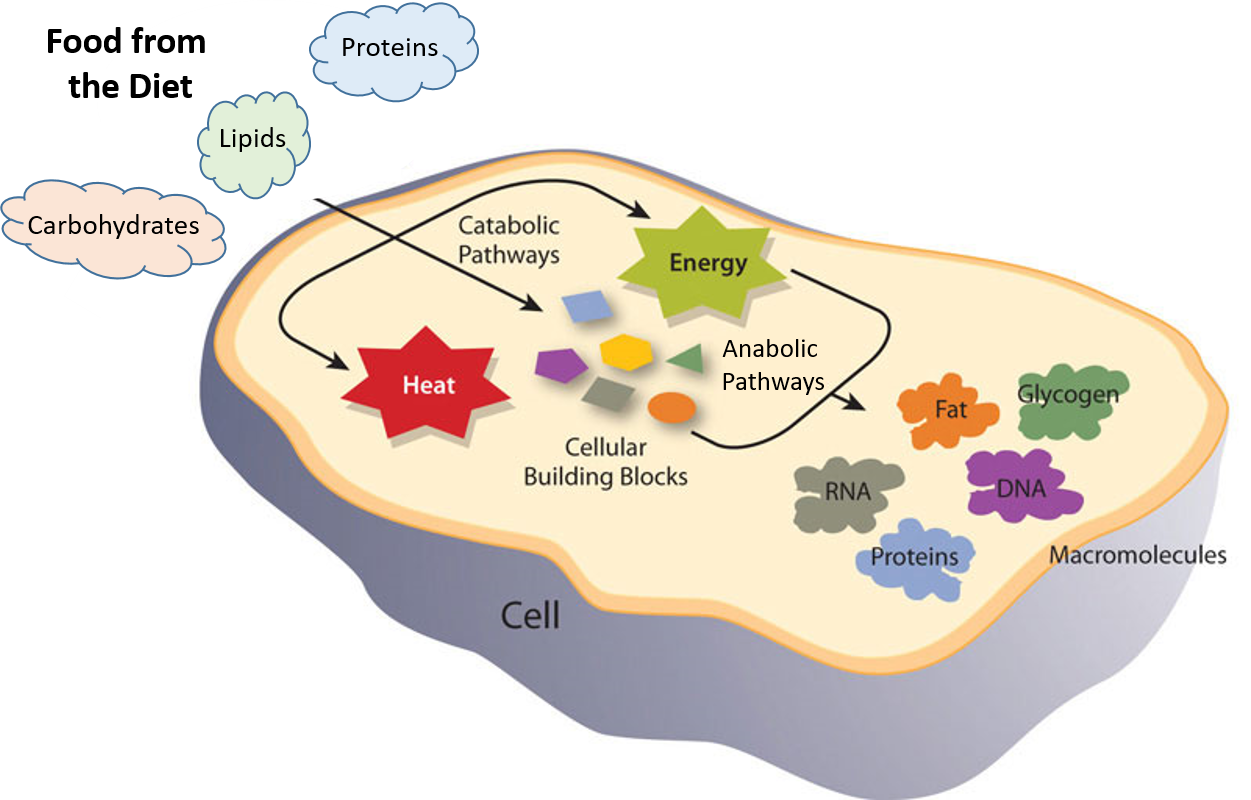
Ch103 Chapter 7 Chemical Reactions In Biological Systems Chemistry
Usually in the presence of an enzyme the reaction runs in the same direction as it would without the enzyme just more quickly.
. The entropy of a system is the degree of random-ness. To predict the effect of temperature on a variety of. Enthalpy entropy and free energy.
The First Law of Thermodynamics also known as the law of conservation of energy states that energy can neither be created nor. Biochemical reactions are usually assumed to proceed at given pH and ionic strength although protons and other ions can be consumed or produced during the reaction. 1to understand the relationship between quantities of heat and work in biological systems.
Thermodynamics of biochemical reactions. These can be articulated in a variety of ways but for our purposes. It can be used to calculate the heat of reaction if the change in.
Energy is neither lost nor created. Thermodynamics of Biochemical Reactions emphasizes the fundamental equations of thermodynamics and the application of these equations to systems of biochemical reactions. 2to understand the influence of energy changes in biological phenomena.
With these tools thermodynamics can be used to describe how systems respond to changes in their environment. EntropyEntropy is the measure. Objectives of thermodynamics All chemical physical and biological processes are ultimately enabled and regulated by the laws of thermodynamics.
This emphasis leads to new thermodynamic potentials that provide criteria for spontaneous change and equilibrium under the conditions in a living cell. The laws of thermodynamics are important unifying principles of biology. It is the H S and G that is considered.
Chemical equations are written in terms of specific ionic and molecular species and balance elements and charge biochemical equations are written in terms of reactants sum of species that consist of species in equilibrium with each. This emphasis leads to new thermodynamic potentials that provide criteria for spontaneous change and equilibrium under the conditions in a living cell. The H of a reaction can be determined from the sum of the Hf of the products minus the Hf of the reactants.
Two types of equations are used to describe chemical and biochemical reactions. Thermodynamics has long been a key theory in biology used in problems ranging from the interpretation of binding both in vitro and in vivo to the study of the conformations of DNA whether under the action of optical traps in well-characterized solutions or in the highly compacted state of the cellular interior. Reaction in which an electron is taken from one atom or molecule and donated to another.
Biochemistry and Molecular Biology Education 321444-444. Thermodynamics of Biochemical Reactions emphasizes the fundamental equations of thermodynamics and the application of these equations to systems of biochemical reactions. In thermodynamics there are two very important topics.
As a consequence the existence of two categories of thermodynamics based on different concepts and different formalisms is now established. As all catalysts enzymes do not alter the position of the chemical equilibrium of the reaction. However in the absence of the enzyme other possible uncatalyzed spontaneous reactions might lead to different products because in.
Enthalpy is the heat content. Biochemical thermodynamics does likewise when it creates new parameters such as G the free energy at a fixed pH. Law that states that the amount of energy in the universe is constant.
The First Law of Thermodynamics. Describes the branch of chemistry that explores the laws that govern energy changes. Entropy and Free energy sometime known as Gibbs free energy or del G In biology specifically these two concepts controls biochemical reactionslets describe it.
Thermodynamics is a system of thinking about interconnections of heat work and matter in natural processes like heating and cooling materials mixing and separation of materials and of particular interest herechemical reactions. Some simple models for solutions can be me made. Gibbs-Helmholtz equation in chemistry a thermodynamic formula that relates changes in the free energy and heat of reaction that take place during chemical reactions.
All cells use a molecule called ____ to carry and release energy cyclically. The first law of thermodynamics says that the energy of a closed system is constant. All graphical constructions derived for the quantity of a mixture can be used for a mixing quantity with appropriate adjustment of standard reference states.
Biochemical reactions of phosphate and sulfate esters are ubiquitous in the living world and are found throughout many pathways involving metabolism biosynthesis control of transcription energy storage and replication. I the chemical thermodynamics which makes use of the conventional thermodynamic properties and it is suitable to deal with only chemical reactions. The amount of energy in the universe is constant in the long run Second LawWhen energy is converted from one form to another within a system less of the originaly available energy is available to do work within that system.
The Laws of Thermodynamics govern biochemical reactions First Law. Thermodynamic concepts are freely used. The second law of thermodynamics says that the entropy in a closed system increases.
Ideal solution regular solution. Ii the biochemical thermodynamics which makes use of. These principles govern the chemical processes metabolism in all biological organisms.
And Mcs logf Af Hf HA were used to. Alberty goes on to use additional Legendre transforms for instance when considering reactions involving coenzymes in which it is assumed that coenzyme concentrations remain constant during a reaction. CH 4 CH 3 H BDECH 3-H.
The ones of immediate relevance to biochemistry are the first and second laws. This can be applied to a wide variety of topics in science and engineering such as engines phase transitions chemical reactions transport phenomena and even black holes. Metabolism is quantified using principles of thermodynamics.
In chemical reactions the ______ stored in the chemical bonds of a molecule can be used to make new bonds in a different molecule.

Biochemical Thermodynamics Applications Of Mathematica Biochemical Thermodynamics Molecular Biology
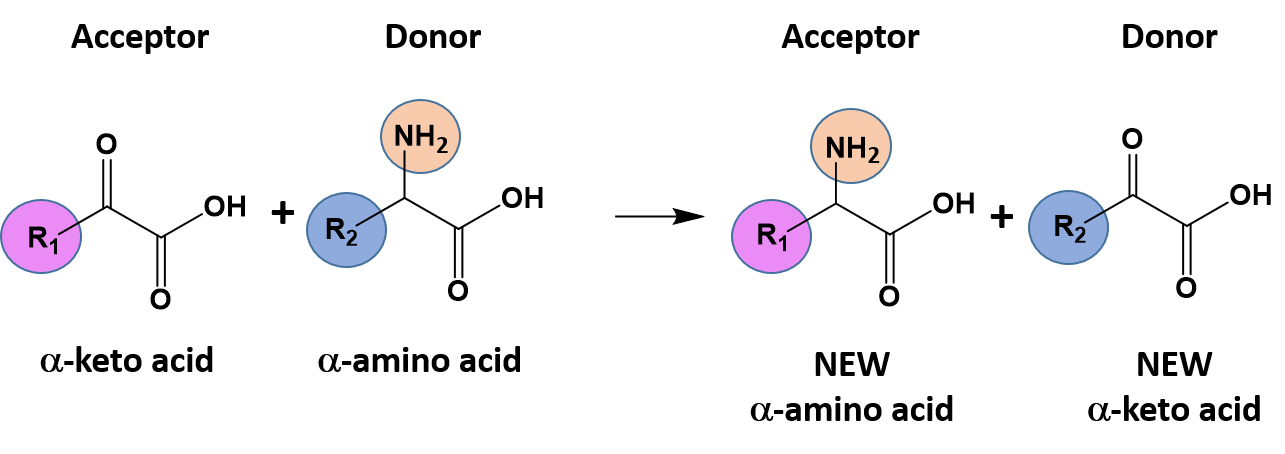
Ch103 Chapter 7 Chemical Reactions In Biological Systems Chemistry

Pin By Raj Guru On Trtrtry Thermodynamics Biochemical Energy

Thermodynamics Thermodynamics Nanotechnology Undergraduate Program
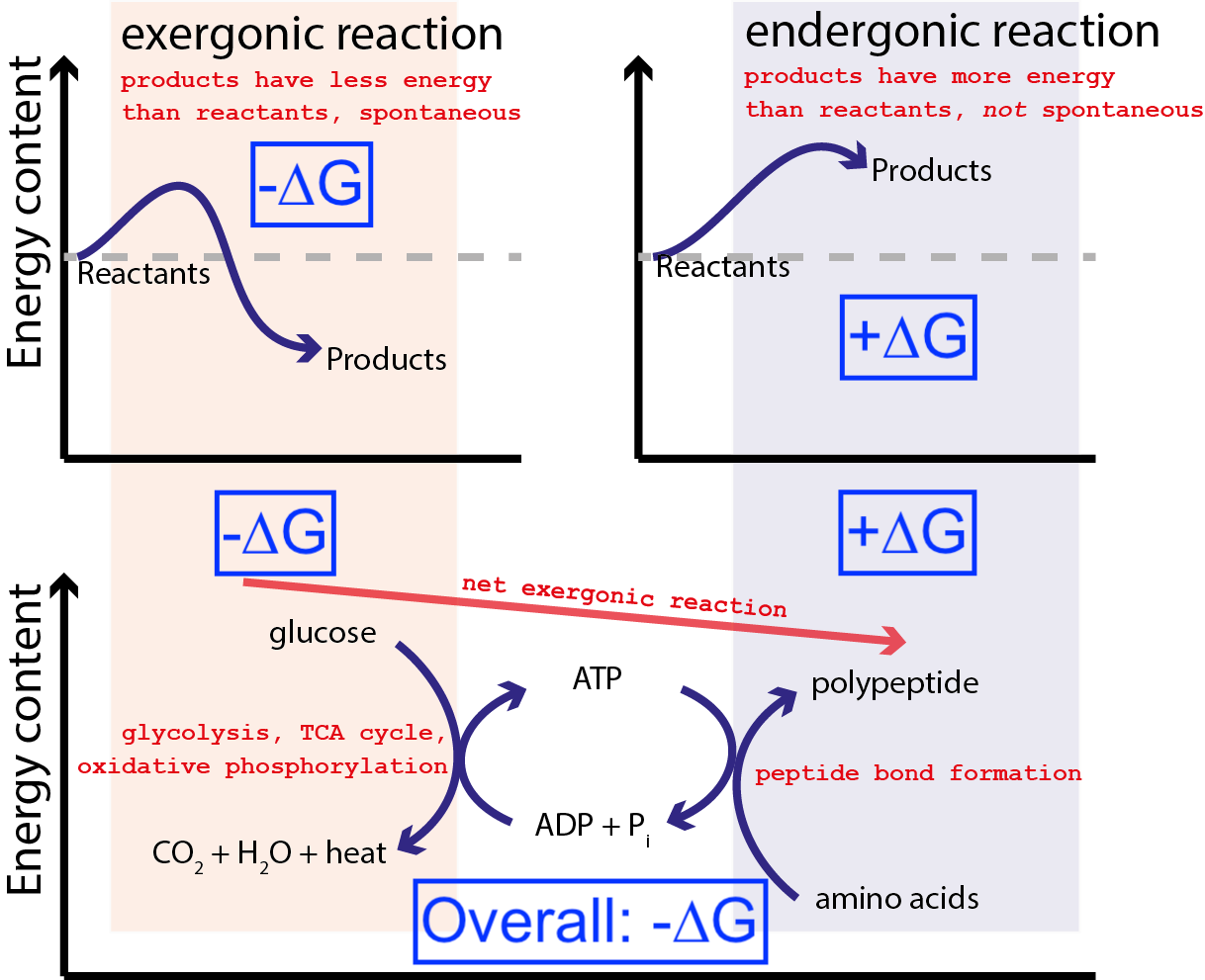
Why G H T S Is Most Important Equation In Biochemistry Big Think

Irjet Design Of Bioreactor For Production Of Methane By Rhodospirillum Nursing Student Tips Chemical Engineering Biochemical Engineering

Introduction To The Chemistry Of Food Paperback Walmart Com In 2021 Chemistry Chemistry Textbook Food Chemistry

Metabolic Biochemistry Pathways Poster Etsy In 2021 Biochemistry College Classes Biochemical

Solved Which Of The Following Accurately Describes The Chegg Com

Phd Position Modeling Of Electrolyte Systems Dtu 2021 Biochemical Engineering Positivity Phd

Pdf Thermodynamics And Biological Systems

Bio And Chem Section Mcat Study Mcat Biochemical

Understanding Enzymes Mcat Study Chemistry Professor Membrane

Physical Chemistry Thermodynamics And Kinetics Thermodynamics Structure And Change Physical Chemistry Chemistry 10 Chemistry Textbook

Bioreaction Engineering Principles 3rd Edition By John Villadsen Jens Nielsen Gunnar Liden Paperback In 2022 Engineering Student Textbook Research Field

Comments
Post a Comment